
In the more than seventy years that we have been operating as the North West’s premier metal finishing company, copper plating has consistently been one of our most popular processes. With thousands of applications across multiple industry sectors, we have been providing hundreds of customers across the UK with our high-quality copper electroplating services.
What is copper plating?

Simply put, copper plating is the process of applying a layer of copper to a base material, using an electrolytic cell, much the same as any other electroplating technique. There are a couple of differences that make the process unique, such as the ability to produce a thicker deposit layer than might normally be expected.
How does copper plating work?
Copper plating is an electrolytic process. The base material and a copper rod are placed into a tank of water with an ionic solution. By the application of a direct current, this allows the copper to ionise, resulting in the loss of an electron by each copper atom, making them positively charged.
These minute particles of copper break off from the rod and dissolve in the solution. The flow of the electric current transfers these copper particles from the copper rod (the anode in this circuit) to the base material in the tank (acting as the cathode).
As it touches the material to be plated, each particle gains an electron, adhering to the material and depositing a thin layer of copper. The thickness of the layer can be adjusted by increasing the length of time the process runs for.

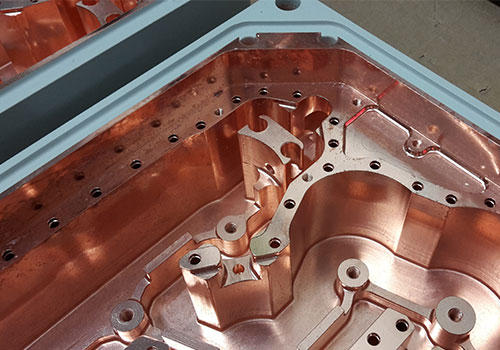
Acid or cyanide?
Here at Karas Plating, we provide cyanide copper and acid copper coatings for our clients. While both produce an effective copper coating, the two techniques have different properties.
Acid copper is generally used where a heavier coating is required, or when a level deposit of copper is essential. It tends to be softer than cyanide copper, with a brighter finish that makes it a good choice for polishing or machining.
Acid copper does have some limitations, however. Because of its high sulphuric acid content, it cannot be directly applied to steel or zinc, though there are ways around this (applying an initial layer of cyanide copper to act as a barrier, for instance).
If you’re not sure which method is best for you, speak to a member of our team. We’ll be only too happy to help.
Applications of the copper plating process
One of the most versatile metals on the planet, copper plated materials have thousands of uses in hundreds of industries around the UK. Copper electroplating is particularly useful in sectors wherever conductivity, corrosion control, adhesion, or thermal management are important.These include, but are certainly not limited to: aerospace, electronics, laboratory/medical, heating, industrial, and electrical.


Benefits of the copper plating process
Copper plating has many benefits compared to other electroplating processes. For starters, it is highly conductive. Indeed, only silver has been shown to have greater conductive properties than copper under standardised testing. It is also a very versatile metal.
The electroplating process produces a smooth and even surface when applied to other metals. This creates a perfect base for primers, paints, and glues. Similarly, the metal is soft and easily malleable, which means that it can be bent, whilst still retaining its inherent adhesive qualities.
Copper electroplating provides a highly conductive, thermally efficient surface, making it ideal for increasing the performance of busbars, connectors, PCBs and other electrical components.
As well as paints and primers, copper serves as the ideal surface on which to add further layers of electroplating.
While not as effective as some harder metals at resisting corrosion, copper is no slouch in this area either.It provides some corrosion resistance and anti galling behaviour, especially on threaded components such as oil and gas pipe threads.
That said, we would recommend using copper as the base to a further layer of nickel plating if corrosion resistance is a key aspect of your metal plating needs. In multi‑layer plating systems, copper is often used as an adhesion‑promoting underlayer that improves bond strength and surface levelling, allowing precious‑metal topcoats to achieve performance with reduced thickness Finally, unlike noble metals like gold, platinum, and silver, copper is in abundant supply and considerably more cost-effective to use.
Latest Resources







